Garrett Research Group: Research Themes
Images


Corrosion of metals and alloys
Corrosion is a deterioration in material properties as a result of chemical reactions with components in the local environment. Common examples include the rusting of iron in humid air or tarnishing of silver in the presence of atmospheric sulfur compounds. Corrosion is necessarily a surface phenomenon and can often be described in terms of reduction and oxidation.
My students and I study two kinds of materials. Metallic alloys known as metallic glasses have unique properties due to their amorphous or glassy internal microstructure. This confers a range of desirable attributes: they are strong, tough, hard and, in principle, corrosion resistant. While most of the research in metallic glasses has been focussed on their synthesis and mechanical characteristics, their corrosion behavior is less well known. We are interested in Cu-based and Pd-based metallic glasses (made in collaboration with Dr. Dale Conner, CSUN Engineering) in acids, bases and high ionic strength electrolyte solutions. We use microscopy, surface-sensitive spectroscopy and electrochemical measurements to characterize their corrosion resistance and the corrosion products formed.
The second type of material are based on metals of importance to cultural heritage, metals and alloys such as bronze and silver. In these studies we are characterizing the surfaces of these materials after exposure to sulfur compounds such as hydrogen sulfide. Hydrogen sulfide is a toxic gas present at very low concentrations in the atmosphere, a result of natural and anthropgenic activities, and is one of the gases responsible for the tarnishing of silver and sterling silver. In collaboration with the J. Paul Getty Museum, we are investigating the reactions that lead to tarnishing and treatments for reversing the effects. Hydrogen sulfide is also present in certain soils. Soil hydrogen sulfide is generated by anaerobic bacteria and can interact with buried ancient bronze artifacts, coating them with a dark copper sulfide patina. We are interested in understanding whether the black patinas observed on some bronze objects in museums could have been generated by such exposure.
Images


Nanoparticle synthesis and reactivity
Nanoparticles are solids with at least one dimension in the 1-100 nm range. They have become a very active research area because some nanoparticles show size-dependent chemical and/or physical properties. The behavior is caused by so-called quantum size effects and it raises the possibility that desirable properties can be created simply by changing the size of the particles.
Our research group studies nanoparticles from two directions. One type of nanoparticle is synthesized by evaporating a metal through a mask layer deposited on a supporting surface. The mask is formed from self-assembled polystyrene spheres and the metal islands formed have triangular shapes. We oxidize the metal to form metal oxide nanoparticles that are adhered to a support. The current solid of interest is titanium dioxide because of its interesting photocatalytic properties, especially when doped with other metals.
A second route to nanoparticles is being explored in conjunction with Dr. Yann Schrodi (CSUN, Chemistry & Biochemistry). In this method, inorganic synthesis is used to make ruthenium nanoparticles about 30 nm in diameter. The nanoparticles are supported and dispersed on silica gel and catalytically-active functional groups are attached to the Ru particle surface. The nanoparticles are air-sensitive which makes their synthesis and measurement challenging. However, there is evidence that they possess good catalytic properties, particularly for the economically-important olefin metathesis-type reactions.
Images
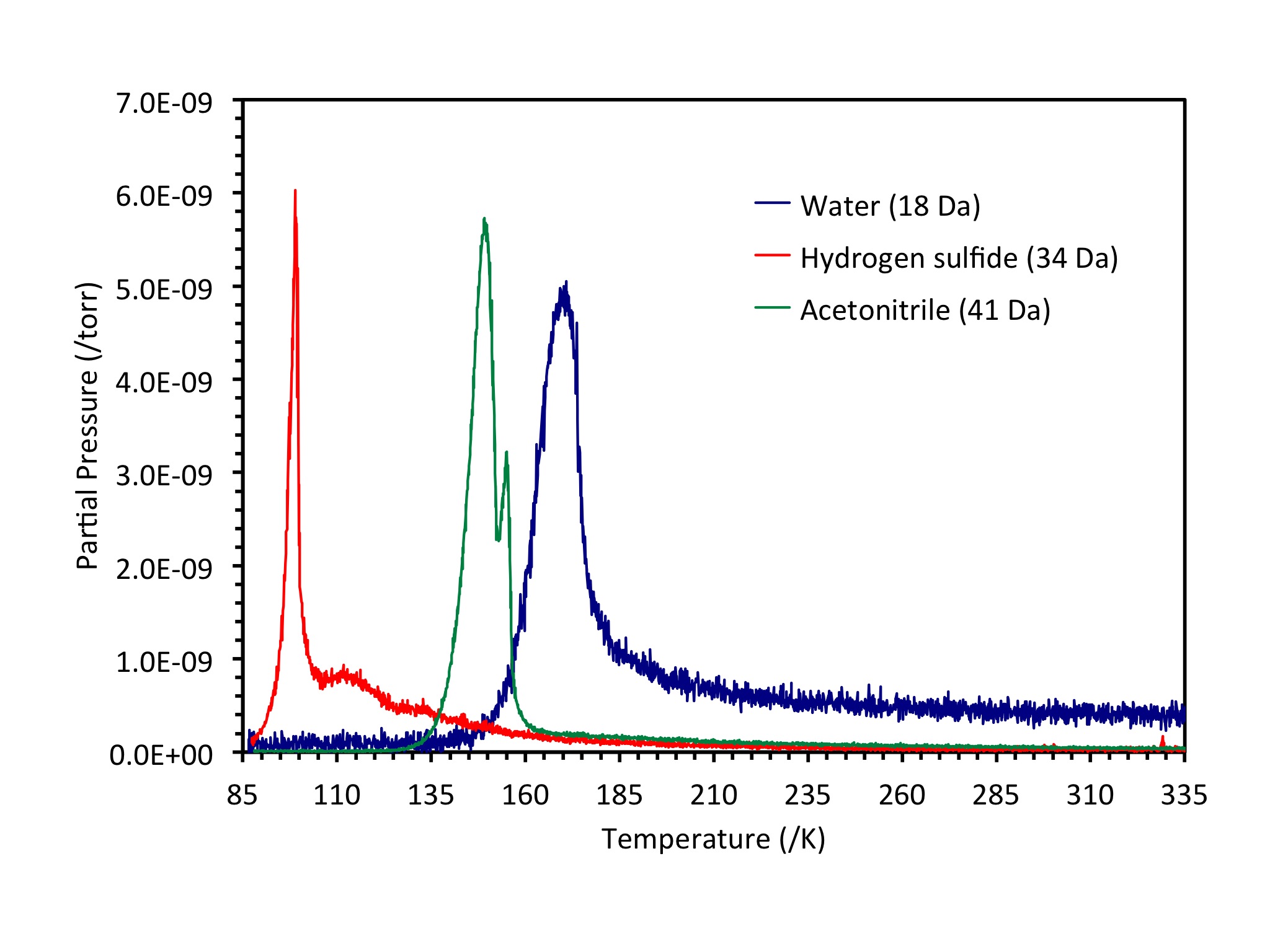
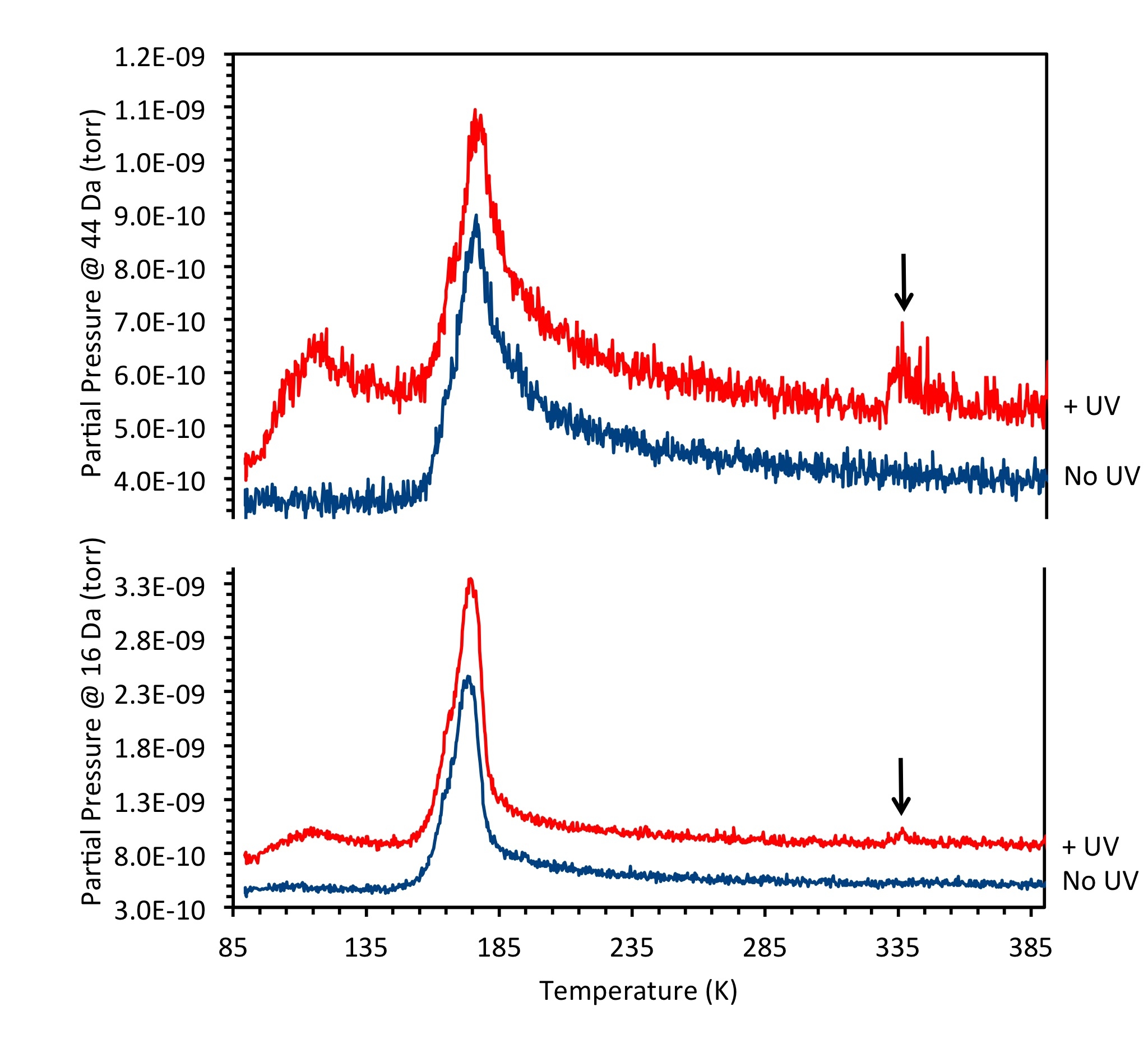
Chemistry in icy layers relevant to astrochemistry
A few dozens of small molecules have been identified in the interstellar medium, including familiar ones such as hydrogen, ammonia, carbon monoxide and water. But the interior of some meteorites, called carbonaceous chondrites, contain thousands of different molecules, some of which are very large and may include aromatic molecules, polymers and even amino acids. The question that immediately arises is how did such complex molecules form in the generally cold, dark regions of the universe?
Our research in this area is aimed at understanding some of the basic chemistry that can take place in cold layers of candidate small molecules (ices) when irradiated by energetic particles such as electrons or UV photons. Electrons are a component of the solar wind and photons are generated by stars. We prepare controlled-composition mixtures of small molecules deposited on a low temperature substrate inside a vacuum chamber, then irradiate with electrons or UV photons. After some period of time, the ice is evaporated by raising the temperature and the products of any reactions collected by a mass spectrometer. We are particularly interested in sulfur-containing molecules and their potential reactions with ammonia, acetonitrile and water. The mass spectrum allows us to identify the products.
The formation of large molecules from small ones in extraterrestrial conditions has a significant impact on our understanding of how life developed on Earth. Was the chemical synthesis of the first biomolecules in the "primordial soup" here on Earth? Were these molecules made outside Earth and then deposited by comets and meteorites? This work is supported by NSF grant #CHE-1464843.
