|
Detailed Explanation of Discrepant
Event |
Principles illustrated
- Colloidal property of milk
- Practical uses of food products
- Visual example of chemical change
|
- Most students think that milk is a simple white liquid, if pressed, they might say it is a solution.
- This discrepant event vividly shows the colloidal property of milk which is a tough concept to get across.
- Milk is mostly water. The protein content is 80% casein protein, 20% whey protein. Milk also has calcium, other minerals, vitamins, butterfat and lactose.
- In it's natural state milk is negatively charged. This negative charge allows for the dispersion of casein in the milk as very small micelles.
- When acid is added to the milk, the positive ions neutralize the negatively charged casein micelles.
- This forms acid casein
- Even though it is no longer used to make buttons, acid casein is still used in the chemical industry and as a glazing additive in paper manufacturing and glue.
- Proteins have distinct physical structures, and are usually folded up based on their charged and hydrophobic residues.
- Denaturing, taking a protein out of its native state, causes proteins to unfold and rearrange which usually causes precipitation or coagulation.
- Caseins are fairly insoluble in water, hence the formation of micelles, and are very insoluble at acid pH.
- Heat and acid are two of the factors that can lead to the aggregation of casein. In this demonstration we heated the milk and added acidic vinegar to cause a chemical reaction.
- We made a natural plastic as the caisson molecules are associated together in long chains.
- This type of plastic is too expensive due to the cost of raw materials, that is why petroleum products are used.
- Cheese is made in a similar fashion. Rennet, an enzyme in a cow's stomach, is usually used to make cheese.
|
|
Standards
- Acids and Bases
- Acids, bases, and salts are three classes of compounds that form ions in water solutions. As a basis for understanding this concept:
- Students know the observable properties of acids, bases, and salt solutions.
- Students know acids are hydrogen-ion-donating and bases are hydrogen-ion-accepting substances.
Solutions
- Solutions are homogeneous mixtures of two or more substances. As a basis for understanding this concept:
- Students know the definitions of solute and solvent.
Reactions
- Chemical reactions are processes in which atoms are rearranged into different combinations of molecules. As a basis for understanding this concept:
- Students know reactant atoms and molecules interact to form products with different chemical properties.
e. Students know how to determine whether a solution is acidic, basic, or neutral.
|
Materials:
- 500ml 2% milk
- 60ml vinegar
- Strainer
- Aluminum foil
- Thermometer
|
Questioning Script
Prior knowledge & experience:
- Proteins are complex polymers composed of amino acids.
- They are the primary structure of all living things.
- Plastics can be either natural or synthetic.
- A plastic is a material that contains as an essential ingredient one or more organic polymeric substances of large molecular weight, is solid in its finished state, and, at some stage in its manufacture or processing into finished articles, can be shaped by flow.
- "Kolloid" was the ancient Greek word for glue.
- Today, a colloid is simply a suspension of small particles in a medium.
- Milk is a colloid because it is a suspension of milk fat globules in water, and so is paint, a suspension of solid pigment granules in oil or water.
Root question: How will milk be affected by the addition of an acid like vinegar?
Target response: Milk is a colloid with proteins suspended in it. The vinegar will denature the protein casein which will polymerize into a natural plastic.
Common Misconceptions:
- Milk is a simple solution
- Milk is a pure liquid
- Milk is not a complex liquid that used in the creation of cheeses and other food products.
|
|
The nice thing about this demonstration is that it can be done at home for an informal lesson too. The supplies needed are shown at the right. |
|
Image of B-Casein. one of the four major casein molecules.
|
|
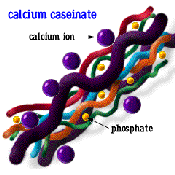
Calcium casienate |
|
Video one is of the straining procedure to separate the new plastic from the liquid whey.
The Second video show the pliable nature of the wet plastic as it is placed on aluminum foil to dry. |
References & Links:
Below is link to similar lab and great deal of background information on this discrepant event
http://www.accessexcellence.org/AE/AEPC/IFT/unit_three.html
Link to definition of a plastic
www.delstarinc.com/glossary.html
Website with colloid definition and other details on colloids
http://scienceweek.com/2003/sc031031-2.htm
This site below also has solid information about dairy science
http://www.ratlab.co.uk/plasticwhy.htm
Here is a detailed list of different man-made and natural plastics. This site also supplied the picture above of the supplies needed.
http://nobel.scas.bcit.ca/resource/plastic/plastic.htm
The following site list many denaturing processes in food, the protein used, and the product.
http://food.oregonstate.edu/learn/milk.html
The below website discusses how to make different cheeses at home.
http://schmidling.netfirms.com/making.htm
The website below from Tillamook Cheese discusses industrial cheese production.
http://www.tillamookcheese.com/education/making.html
|