Kubic Bubbles
SED 695B
Advanced Laboratory Curriculum Developement
Principles
illustrated:
* symmetry
*shortest distance between two points
*minimum surface area
*Helmholtz free energy

Standards addressed:
9a&b. Scientific progress is made by asking meaningful
questions and conducting careful investigations. As a basis for understanding
this concept and addressing the content in the other three strands,
students should develop their own questions and perform investigations.
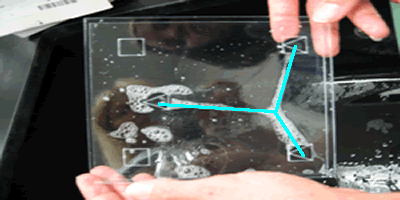
*two 13cm x 13cm plexiglass plates
*four rubber suckers
*bubble solution
This is the result of minimizing Helmholtz free energy. Helmholtz free energy in the internal energy of a system which is based on the internal energy, entropy and temperature of the system. The Helmholtz free energy is a measure of the amount of energy you have to put in to create a system once the spontaneous energy transfer to the sytem from the environment is accounted for.
Procedure:
* pour bubble solution into a shallow pan large enough to accomodate
the plexiglass plates
*place rubber suckers in desired position (triangle, square, etc)
* dip the plates into the bubble solution
*withdraw them nearly horizontally
*tip them gently to allow the water to drain off until soap film settles
in one position


References & Links
Helmholtz Free Energy Explanation
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/helmholtz.html
Calculating Surface Area
http://regentsprep.org/Regents/Math/farea/TArea.htmhttp://regentsprep.org/Regents/Math/farea/TArea.htm