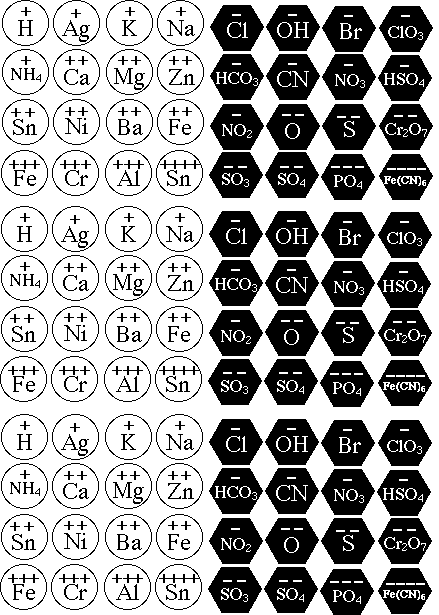
- aluminum Al3+
- ammonium NH4+
- barium Ba2+
- calcium Ca2+
- chromium(III) Cr3+
- cobalt(II) Co2+
- copper(I) Cu+
- copper(II) Cu2+
- hydronium H3O+
- iron(II) Fe2+
- iron(III) Fe3+
- lead(II) Pb2+
- magnesium Mg2+
- mercury(I) Hg22+
- mercury(II) Hg2+
- nickel(II) Ni2+
- potassium K+
- silver Ag+
- sodium Na+
- tin(II) Sn2+
- tin(IV) Sn4+
- zinc Zn2+
|
- acetate (C2H3O)2-
- bromide Br-
- carbonate CO32-
- chlorate ClO32-
- chloride Cl-
- chlorite ClO2-
- chromate CrO42-
- cyanide CN-
- dichromate (Cr2O7)2-
- fluoride F-
- hexacyanoferrate(II) Fe(CN)64-
- hexacyanoferrate(III) Fe(CN)63-
- hydride H-
- hydrogen carbonate HCO3-
- hydrogen sulfate HSO4-
- hydroxide OH-
- hypochlorite ClO-
- iodide I-
- nitrate NO3-
- nitrite NO2-
- oxide O2-
- perchlorate ClO4-
- permanganate MnO4-
- peroxide O22-
- phosphate PO43-
- sulfate SO42-
- sulfide S2-
- sulfite SO32-
|