Temperature Probes & Heating/Cooling Curves
SED 695B; Fall 2005
Research Question: What does the temperature vs. time plot look like for a substance undergoing a phase change?

Standards addressed:
H.S. Chemistry
- 6f.* Students know how molecules in a solution are separated or purified by the methods of chromatography and distillation.
- 7c. Students know energy is released when a material condenses or freezes and is absorbed when a material evaporates or melts.
- 7d. Students know how to solve problems involving heat flow and temperature changes, using known values of specific heat and latent heat of phase change.
- Vernier Lab Pro Interface
- Computer
- Logger Pro Software
- Temperature Probe
- Lauric Acid
- Isopropyl Alcohol (70% or 90%)
- Distilled Water
- Hot Plate
Isopropyl Alcohol & Water
- Make a mixture containing approximately 50% Isopropyl Alcohol and 50% Distilled water.
- Place in a flask or test tube.
- Place three or four porcelain chips in the container to moderate bubbling during boiling.
- Stopper the container with a hole for gas to escape and a hole for the temperature probe.
- Ensure that the probe or the thermometer extends into the container but above the mixture.
- Heat slowly until the mixture boils recording the temperature at regular intervals.
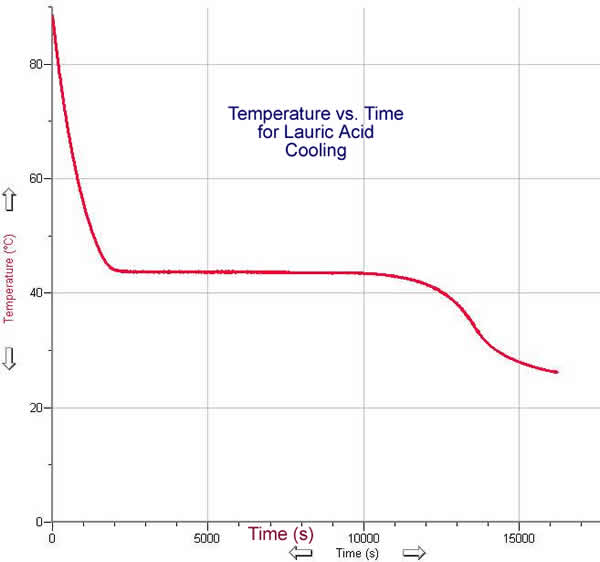
Lauric Acid
- Fill a 250 mL beaker or other container approximately 3/4 full with Lauric Acid.
- Place the temperature probe in the middle of the acid flakes without touching the sides or bottom.
- Heat the acid until it melts completely.
- Adjust the data range so that data is taken during the entire time the acid cools and solidifies.
- Remove the heat and begin taking data.
- Clean container with methanol or benzene.
Caution:
The mixture is flammable and any that boils out of the container can burst into flame. The mixture can and will vigorously boil. Our suggestion is to not use a bunsen burner.
Smaller containers such as a 25mm x 150mm test tube require only a very small heat source such as an alcohol burner. Bunsen burners, even adjusted to a low setting, easily boil out of the test tube. Even with an alcohol burner, care must be taken through adjustment of the position of the burner to slowly boil the mixture.
Larger containers such as a 250 mL flask can be heated with a hot plate more easily and safely than with a bunsen burner.
Lauric Acid is a skin irritant. See the material safety data sheet below.
Blank Data Table |
|
Time (s) |
Temperature(Celsius) |
Sample Data Table |
|
Time (s) |
Temperature(Celsius) |
10 |
25 |
20 |
30 |
30 |
35 |
40 |
35 |
50 |
35 |
60 |
35 |
| Time vs. Temperature for a Heating Mixture of Water & Isopropyl Alcohol | |||||||
| Time (s) | Temp(°C) | Time (s) | Temp(°C) | Time (s) | Temp(°C) | Time (s) | Temp(°C) |
| 0.0 | 46.9 | 812.5 | 81.9 | 1625.0 | 83.6 | 2763.0 | 99.6 |
| 12.5 | 47.3 | 825.0 | 81.9 | 1637.5 | 83.7 | 2775.5 | 99.7 |
| 25.0 | 47.8 | 837.5 | 81.9 | 1650.0 | 83.8 | 2788.0 | 99.6 |
| 37.5 | 48.4 | 850.0 | 81.9 | 1662.5 | 83.9 | 2800.5 | 99.7 |
| 50.0 | 48.9 | 862.5 | 81.9 | 1675.0 | 84.0 | 2813.0 | 99.6 |
| 62.5 | 49.7 | 875.0 | 81.9 | 1687.5 | 84.1 | 2825.5 | 99.6 |
| 75.0 | 50.6 | 887.5 | 81.8 | 1700.0 | 84.1 | 2838.0 | 99.7 |
| 87.5 | 51.6 | 900.0 | 81.9 | 1712.5 | 84.3 | 2850.5 | 99.6 |
| 100.0 | 52.7 | 912.5 | 81.9 | 1725.0 | 84.4 | 2863.0 | 99.6 |
| 112.5 | 53.9 | 925.0 | 82.0 | 1737.5 | 84.5 | 2875.5 | 99.6 |
| 125.0 | 55.4 | 937.5 | 82.0 | 1750.0 | 84.7 | 2888.0 | 99.7 |
| 137.5 | 57.2 | 950.0 | 82.0 | 1762.5 | 84.7 | 2900.5 | 99.6 |
| 150.0 | 60.2 | 962.5 | 82.1 | 1775.0 | 84.9 | 2913.0 | 99.6 |
| 162.5 | 65.5 | 975.0 | 82.1 | 1787.5 | 85.1 | 2925.5 | 99.6 |
| 175.0 | 71.2 | 987.5 | 82.1 | 1800.0 | 85.2 | 2938.0 | 99.7 |
| 187.5 | 75.3 | 1000.0 | 82.1 | 2138.0 | 90.7 | 2950.5 | 99.6 |
| 200.0 | 78.8 | 1012.5 | 82.1 | 2150.5 | 91.1 | 2963.0 | 99.7 |
| 212.5 | 80.8 | 1025.0 | 82.1 | 2163.0 | 91.6 | 2975.5 | 99.6 |
| 225.0 | 81.3 | 1037.5 | 82.1 | 2175.5 | 92.1 | 2988.0 | 99.7 |
| 237.5 | 81.6 | 1050.0 | 82.2 | 2188.0 | 92.6 | 3000.5 | 99.7 |
| 250.0 | 81.8 | 1062.5 | 82.1 | 2200.5 | 93.0 | 3013.0 | 99.7 |
| 262.5 | 81.8 | 1075.0 | 82.1 | 2213.0 | 93.6 | 3025.5 | 99.7 |
| 275.0 | 81.8 | 1087.5 | 82.1 | 2225.5 | 94.1 | 3038.0 | 99.6 |
| 287.5 | 81.8 | 1100.0 | 82.1 | 2238.0 | 94.5 | 3050.5 | 99.6 |
| 300.0 | 81.9 | 1112.5 | 82.1 | 2250.5 | 95.2 | 3063.0 | 99.7 |
| 312.5 | 81.9 | 1125.0 | 82.2 | 2263.0 | 95.7 | 3075.5 | 99.6 |
| 325.0 | 81.9 | 1137.5 | 82.3 | 2275.5 | 96.1 | 3088.0 | 99.7 |
| 337.5 | 81.9 | 1150.0 | 82.3 | 2288.0 | 96.6 | 3100.5 | 99.6 |
| 350.0 | 81.9 | 1162.5 | 82.3 | 2300.5 | 96.9 | 3113.0 | 99.7 |
| 362.5 | 81.8 | 1175.0 | 82.3 | 2313.0 | 97.3 | 3125.5 | 99.7 |
| 375.0 | 81.9 | 1187.5 | 82.3 | 2325.5 | 97.7 | 3138.0 | 99.7 |
| 387.5 | 81.9 | 1200.0 | 82.3 | 2338.0 | 98.0 | 3150.5 | 99.6 |
| 400.0 | 81.9 | 1212.5 | 82.3 | 2350.5 | 98.2 | 3163.0 | 99.7 |
| 412.5 | 81.9 | 1225.0 | 82.3 | 2363.0 | 98.3 | 3175.5 | 99.7 |
| 425.0 | 81.9 | 1237.5 | 82.4 | 2375.5 | 98.5 | 3188.0 | 99.7 |
| 437.5 | 81.9 | 1250.0 | 82.4 | 2388.0 | 98.7 | 3200.5 | 99.7 |
| 450.0 | 81.9 | 1262.5 | 82.4 | 2400.5 | 98.8 | 3213.0 | 99.6 |
| 462.5 | 81.9 | 1275.0 | 82.4 | 2413.0 | 98.9 | 3225.5 | 99.6 |
| 475.0 | 81.9 | 1287.5 | 82.4 | 2425.5 | 99.0 | 3238.0 | 99.7 |
| 487.5 | 81.8 | 1300.0 | 82.5 | 2438.0 | 99.1 | 3250.5 | 99.7 |
| 500.0 | 81.9 | 1312.5 | 82.5 | 2450.5 | 99.2 | 3263.0 | 99.6 |
| 512.5 | 81.9 | 1325.0 | 82.5 | 2463.0 | 99.2 | 3275.5 | 99.7 |
| 525.0 | 81.9 | 1337.5 | 82.5 | 2475.5 | 99.2 | 3288.0 | 99.7 |
| 537.5 | 81.9 | 1350.0 | 82.5 | 2488.0 | 99.3 | 3300.5 | 99.7 |
| 550.0 | 81.9 | 1362.5 | 82.5 | 2500.5 | 99.3 | 3313.0 | 99.7 |
| 562.5 | 81.9 | 1375.0 | 82.6 | 2513.0 | 99.3 | 3325.5 | 99.7 |
| 575.0 | 81.9 | 1387.5 | 82.6 | 2525.5 | 99.5 | 3338.0 | 99.7 |
| 587.5 | 81.9 | 1400.0 | 82.7 | 2538.0 | 99.5 | 3350.5 | 99.7 |
| 600.0 | 81.9 | 1412.5 | 82.7 | 2550.5 | 99.5 | 3363.0 | 99.7 |
| 612.5 | 81.9 | 1425.0 | 82.7 | 2563.0 | 99.5 | 3375.5 | 99.7 |
| 625.0 | 81.9 | 1437.5 | 82.7 | 2575.5 | 99.5 | 3388.0 | 99.7 |
| 637.5 | 81.9 | 1450.0 | 82.8 | 2588.0 | 99.6 | 3400.5 | 99.7 |
| 650.0 | 81.9 | 1462.5 | 82.8 | 2600.5 | 99.5 | 3413.0 | 99.7 |
| 662.5 | 81.9 | 1475.0 | 82.8 | 2613.0 | 99.6 | 3425.5 | 99.7 |
| 675.0 | 81.9 | 1487.5 | 82.9 | 2625.5 | 99.6 | 3438.0 | 99.7 |
| 687.5 | 81.9 | 1500.0 | 82.9 | 2638.0 | 99.6 | 3450.5 | 99.7 |
| 700.0 | 81.9 | 1512.5 | 83.0 | 2650.5 | 99.6 | 3463.0 | 99.7 |
| 712.5 | 81.9 | 1525.0 | 83.1 | 2663.0 | 99.6 | 3475.5 | 99.7 |
| 725.0 | 81.9 | 1537.5 | 83.2 | 2675.5 | 99.6 | ||
| 737.5 | 81.9 | 1550.0 | 83.2 | 2688.0 | 99.6 | ||
| 750.0 | 81.9 | 1562.5 | 83.3 | 2700.5 | 99.6 | ||
| 762.5 | 81.9 | 1575.0 | 83.4 | 2713.0 | 99.6 | ||
| 775.0 | 81.9 | 1587.5 | 83.4 | 2725.5 | 99.6 | ||
| 787.5 | 81.9 | 1600.0 | 83.4 | 2738.0 | 99.6 | ||
| 800.0 | 81.9 | 1612.5 | 83.6 | 2750.5 | 99.6 | ||



References & Links:
- Vernier
- Vernier Lab Pro Interface
- Vernier Logger Pro Software
- Vernier Temperature Probe
- Pasco (Makers of lab equipment)
- Material Safety Data Sheet for Lauric Acid
- Material Safety Data Sheet for Isopropyl Alcohol/Water Solution
- Latent Heat of Fusion and Vaporization