Research Question:
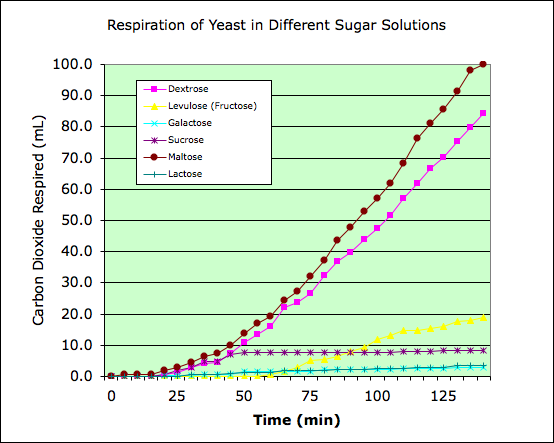
Students are often confused by the term isomer. Eventually, they memorize a definition and know that isomers share atomic composition, but vary in their structures. What are the consequences for organisms? Can organisms use any molecule for energy as long as they have the same chemical formulas? Or does the structure of each molecule affect the usefulness of a molecule? In this investigation, it is determined that not all sugar is the same. Only certain configurations of sugar molecules can be used by yeast.
Three Monomers Shown Below--Three Dimers Shown Above

A Possible Setup

Standards addressed:
Biology/Life Sciences
Cell Biology
1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism’s cells. As a basis for
understanding this concept:
b. Students know enzymes are proteins that catalyze biochemical reactions without
altering the reaction equilibrium and the activities of enzymes depend on the
temperature, ionic conditions, and the pH of the surroundings.
g. Students know the role of the mitochondria in making stored chemical-bond
energy available to cells by completing the breakdown of glucose to carbon
dioxide.
h. Students know most macromolecules (polysaccharides, nucleic acids, proteins,
lipids) in cells and organisms are synthesized from a small collection of simple
precursors.
Investigation and Experimentation
1. Scientific progress is made by asking meaningful questions and conducting careful
investigations. As a basis for understanding this concept and addressing the content
in the other four strands, students should develop their own questions and perform
investigations. Students will:
d. Formulate explanations by using logic and evidence.