Science Kit for Student Use: Polyurethane Foam
Author(s): Peggy_Klipfel_LeDuff
Jim_ Mauch
SED 695B; Fall 2005
Topics addressed:
8th Grade Physical Science
Reactions
5. Chemical reactions are processes in which atoms are rearranged
into different combinations of molecules. As a basis for understanding
this concept:
a. Students know reactant atoms and molecules interact to form
products with different chemical properties.
b. Students know the idea of atoms explains the conservation of matter:
In chemical reactions the number of atoms stays the same no matter
how they are arranged, so their total mass stays the same.
c. Students know chemical reactions usually liberate heat or absorb
heat.


Supplies
1. Polyurethane Foam System (Part A and Part B)
(Order these chemicals through Flinn: http://www.flinnsci.com/).
2. clear,disposable plastic cups (two per lab group)
3. paper towels and newspapers
4. stirring rod
5. food coloring (optional)
6. disposable gloves (clear)
| Simply mix any 50/50 mixture of Part A and Part B to create a hardened, lightweight polyurethane foam which is 30 times the original volume of the two liquid chemicals!!! |
|
Procedure:
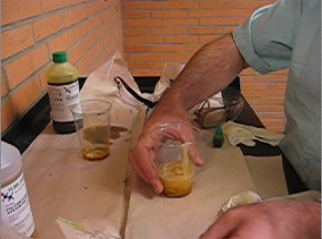
1. In a fume hood or well ventilated area, pour approximately 20 mL of liquid Part A in a disposable cup.
Note: The exact volume is not critical. Add a few drops of food coloring, if desired, and stir.
2. Place approximately 20 mL of liquid Part B in a second disposable cup.
Note: The volume of Part B should be approximately equal to that of Part A.
3. If desired, add several drops of food coloring to one of the cups and stir thoroughly to mix.
4. Spread a paper towel or newspaper flat on the table and place one of the cups in center of the paper towel.
5. Pour the contents from the second cup into the cup on the paper towel and stir thoroughly until you see the foam beginning to expand. Remove the stirring rod.
6. Observe the foam as it expands to approximately 30 times its original volume. The cup will get warm, indicating an exothermic reaction. Do not touch the foam until it is completely hardened.
Questions:
1. Does this experiment create an endothermic or exothermic reaction?
2. What is density?
3. What is the density of Part A chemical and Part B chemical?
4. What is the density of the polyurethane foam that is created?
5. Would this foam be used as a flotation device?
6. What type of chemical reaction occurs during this experiment?
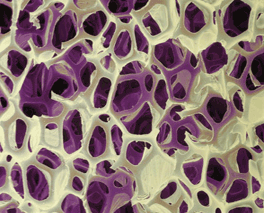
| Click on the photo on the left to view the experiment. The photo on the right is a magnified image of the foam cells created by the foam. |
 |


The Reactants
Part A is a viscous cream-colored liquid containing a polyether polyol, a silicone surfactant, and a catalyst. The hydroxyl end of the polymer is the reactive site. The silicone surfactant reduces the surface tension between the liquids. The catalyst is a tertiary amine which aids in speeding up the reaction without being chemically changed itself.
Part B is a dark brown viscous liquid containing diphenylmethane diisocyanate and higher oligomers of diisocyanate. When the polyether polyol in Part A is mixed with the diisocyanate in Part B, an exothermic reaction occurs, producing polyurethane,a FOAM.

The Product
During the polymerization reaction, a small amount of water reacts with some of the diisocyanate. A decomposition reaction occurs and produces carbon dioxide gas which causes the solution to foam and to expand in volume. Pores in the mixture are created from the gas which are visible when looking at the rigid substance. The high degree of crosslinking in the polymer causes the foam to become rigid in minutes.
Uses* of Polyurethane Foam
packaging
insulation
flotation devices
furniture arms
furniture backs
furniture seat cushions
automotive seat cushions
mattresses cores
carpet underlay
seal holes to prevent uninvited guests into dwellings
*(besides fun foam hands & mushroom foam cups)

 |
Another photograph using the USB microscope. Notice the honeycomb structures that are formed when the Part A and Part B of the science kit are mixed. The honeycomb structures each have 25 planes to provide strength to the foam. |
insulation w/foam |
decorations made of foam |
packaging w/foam |
 safety & comfort w/foam |
Safety Issues
Click here to go to Flinn Science for help with chemical precautions
and clean up.
Helpful websites for chemistry questions:
http://www.chemtutor.com
http://www.csun.edu/chemteach
http://www.boshf.org/chembank
References & Links:
http://www.flinnsci.com/
http://www.chem.purdue.edu/bcce/
http://www.chemistry.org
http://www.shopmanic.com
http://www.pfa.org/