The Copper Test Tube
Forming Metallic Copper by an Oxidation - Reduction Reaction
SED 695B; Fall 2005
Overview:
By simply mixing three solutions in a test tube and heating in a water bath for a few minutes, you will see a thin layer of copper form on the walls of the test tube.

- Oxidation/reduction reactions
- Balancing chemical equations
- Formation of precipitates
Standards addressed:
Conservation of Matter and Stoichiometry
3. The conservation of atoms in chemical reactions leads to the principle of conservation of matter and the ability to calculate the mass of products and reactants. As a basis for understanding this concept:
a.
Students know how to describe chemical reactions by writing balanced equations. g.
* Students know how to identify reactions that involve oxidation and reduction and how to balance oxidation-reduction reactions.
Procedure:
- Roughen the inside of a small test tube with steel wool. This is difficult but important.
- Wash the test tube with a few ml of concentrated nitric acid. Exercise caution with concentrated acids. Use a fume hood and wear face shield and gloves. Rinse with distilled water.
- Rinse with 20 drops of the stannic chloride solution.
- Rinse with 20 drops of the silver nitrate solution
- Mix equal amounts of Fehlings solutions 'a' and 'b' in a beaker. For one demonstration, use 2ml of each.
- Place 4ml of the mixture into the test tube.
- add 1ml of glyoxal solution to the test tube
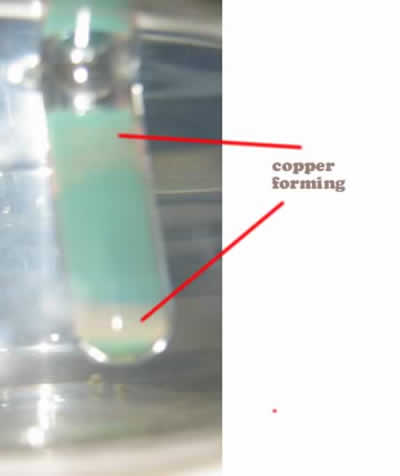
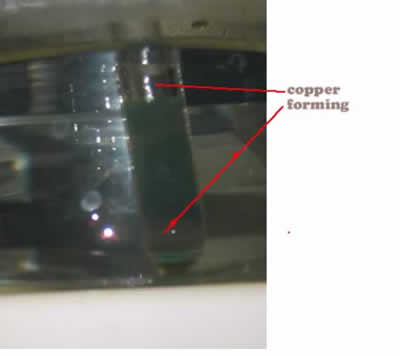
- Heat in warm water bath (50 degrees C) and observe as copper mirror forms.
Description of Chemistry:
![]()
Questions:
Balance the equation for this reaction.
Chemicals used:
Pictured are the chemicals in the kit provided by Flinn Scientific.
- concentrated nitric acid (not shown)
- acidified stannic chloride solution (right)
- silver nitrate solution (left)
- Fehlings solution 'a' (aka Cupric Sulfate solution) (second from left)
- Fehlings solution 'b' (aka Potassium Sodium Tartrate solution) (second from right)
- 40% glyoxal (center)



References & Links:
Expanded experiments on this reaction, including data as to optimal temperatures.
Safety data for glyoxal.
Practical applications:
Similar processes could be used to add the metal plating on glass to make mirrors.
