Change of State in a Pulse Glass
SED 695B; Fall 2005
Principles illustrated:
- Charles' Law
- Change of phase between liquid and vapor in a confined fluid.


Standards addressed:
PHYSICS
Heat and Thermodynamics
3. c. Students know the internal energy of an object includes the energy of random motion of the object's atoms and molecules, often referred to as thermal energy. The greater the temperature of the object, the greater the energy of motion of the atoms and molecules that make up the object.
CHEMISTRY
Gases and Their Properties
4. b. Students know the random motion of molecules explains the diffusion of gases.
d. Students know the values and meanings of standard temperature and pressure (STP).
Chemical Thermodynamics
7. a. Students know how to describe temperature and heat flow in terms of the motion of molecules (or atoms).
EIGHT GRADE PHYSICAL SCIENCE
Structure of Matter
3. d. Students know the states of matter (solid, liquid, gas) depend on molecular motion.
Materials
Pulse Glass
Explanation of principles involved
Charles' Law states that the volume of a given amount of dry ideal gas is directly proportional to the temperature if the amount of gas and the pressure remains fixed. A plot of the volume of a gas against the temperature it forms a straight line. The mathematical statement is that the V / T = a constant.
The pulse glass contains a volatile liquid and vapor sealed in glass container at a low pressure. Heating one bulb with your hand increases the vapor pressure in the bulb, forcing the liquid through a connecting tube into the other bulb in a series of bubbles or pulses.
Procedure:
1. Enclose one bulb of the pulse glass in one hand.
2. Angle the pulse glass slightly so all of the liquid starts off in the bulb you are holding at a lower angle than the empty bulb.
3. After several seconds the heat from your hand will change some of the liquid on the end you are holding to vapor, increasing the pressure in that end and forcing the liquid into the other end.
4. The liquid should move to the other bulb and bubble quite violently for a few seconds.
Warning:
Do not expose the pulse glass to any other source of heat except your hand. Other heat sources, such as burners, can cause the glass to shatter and spread the volatile liquid, causing injuries.
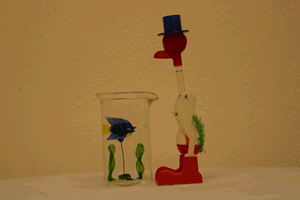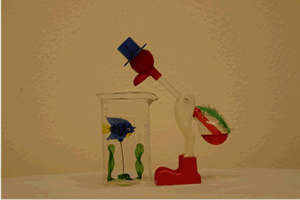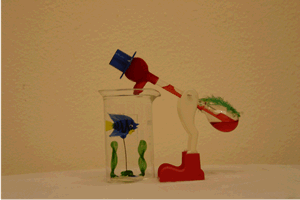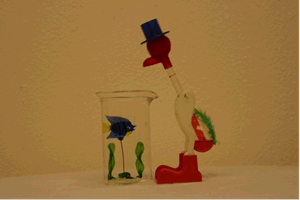
One way to introduce this piece of equipment is with the "Drinking Bird" demonstration. Over a period of several class sessions it could be used as a focus for class discussion. Have the students propose the mechanism behind the bird's continued "drinking".
Of course the bird contains a closed system like the pulse glass. Its head is covered with a cloth which soaks up the water from the beaker. As the water evaporates from the head, it cools the top bulb and allows the room temperature to increase the vapor pressure in the lower bulb until the fluid causes the bird to pivot on his legs and dip his head into the water.
In this video clip one can see the correct position in which to hold the pulse glass.
All of the fluid should start in the lower bulb.
As a hand is placed on the lower bulb, body heat causes the liquid to vaporize.
Increased vapor pressure in the lower bulb forces the liquid into the upper bulb.
Bubbles begin to appear as the liquid continues to vaporize in the lower bulb and after gravity has forced the condensed liquid in the upper bulb to reenter the lower bulb.

Image from the Department of Physics at the University of Maryland
This demonstration could be used to get the students to think about what might be happening to the fluid in the tube that causes the changes that they observe.
Why don't you see fluids in tubes behave this way all the time?
The heat from your hand begins the reaction, why does it continue to pulse?

Image from the Department of Physics at the University of Maryland
References & Links:
CSU San Bernardino
http://chem.csusb.edu/~chem304/facresource/facultyresources.htm#week3
University of Maryland, Physics Department
http://www.physics.umd.edu/lecdem/services/demos/demosi4/i4-18.htm
Boston University, Physics Department
http://buphy.bu.edu/~duffy/thermo/4C33_50.html
Oberlin University, Ohio
http://www.oberlin.edu/physics/catalog/demonstrations/thermo/pulseglass.html
Stony Brook University, New York
http://naples.cc.sunysb.edu/CAS/pdemos.nsf/By+Course+Number/I.+ThermodynamicsI4.+Changes+Of+State+I4-18Pulse+Glass
University of Michigan, Department of Physics
http://www.physics.lsa.umich.edu/demolab/demo.asp?id=793
Standards from CSUN
https://www.csun.edu/science/standards/index.html
World of Chemistry: The Home Page of Ralph Logan
http://members.aol.com/profchm/charles.html